Vaccinating against corona
We present all the information you need about the biggest vaccination campaign in German history, the vaccines themselves and Covid-19 research.

How much progress has been made with vaccinations so far?
The Vaccination Dashboard of the Federal Ministry of Health provides a broad overview of the progress of Covid 19 vaccinations in Germany. At www.impfdashboard.de, you can see how many people have been vaccinated each day, and find out how vaccinations are progressing in the individual target groups.
Who is being vaccinated?
Currently, vaccination is being carried out by mobile teams in nursing homes and at vaccination centres in Germany’s federal states. Vaccinations at doctors' surgeries are not yet possible. Who are in the groups that can receive vaccination first? What is the order of priority?
Group 1 - Highest priority (only this group is currently entitled to a vaccination):
- People over 80 years of age
- People who are treated, cared for or work in inpatient facilities for elderly people or people in need of care
- Caregivers in outpatient care services
- Employees in medical facilities with a high risk of exposure, such as intensive care units, emergency rooms and rescue services; providers of specialised outpatient palliative care; employees working in corona vaccination centres and areas involving potentially infectious activities
- Workers in medical facilities that treat or provide care to people at high risk (especially haemato-oncology and transplant medicine).
Group 2 - High priority:
- People over 70 years of age
- People with Down’s syndrome, dementia or intellectual disability, and those who have received an organ transplant.
- Those in close contact with people over 80 years of age or the residents of nursing homes and homes for the mentally handicapped
- Those in contact with pregnant women
- Persons who work in inpatient facilities for the mentally handicapped or who regularly treat, care for or nurse mentally handicapped persons as part of outpatient care services.
- Persons working in areas of medical facilities with a high or increased risk of exposure to coronavirus, in particular doctors and other staff with regular patient contact, staff of blood and plasma donation services and corona testing centres.
- Police and law enforcement personnel who are at high risk of infection when on duty, such as during demonstrations
- Persons in the public health service and in relevant positions in the hospital infrastructure
- Persons living or working in refugee and homeless facilities.
Group 3 - Increased priority:
- People over 60 years of age
- Persons with the following conditions: obesity, chronic kidney disease, chronic liver disease, immunodeficiency or HIV infection, diabetes mellitus, various heart diseases, stroke, cancer, COPD or asthma, autoimmune diseases or rheumatism.
- Employees in medical facilities with a low risk of exposure (laboratories) and who are not involved in caring for patients with suspected infectious diseases
- Persons in relevant positions in governments, administrative and constitutional bodies, the armed forces, police, fire brigade, disaster control and THW, the judiciary
- Persons in relevant positions in critical infrastructure companies, in the pharmacy and pharmaceutical industry, in public utilities and waste disposal companies, and in the food, transport, information technology and telecommunications sectors
- Educators and teachers
- Persons with precarious working or living conditions
Group 4 - No priority
- All those who have a lower risk of suffering a severe course of Covid 19. They should be offered vaccination according to the prioritised groups.
How were the vaccines approved?
No shortcuts were taken in the approval of the Biontech and Moderna vaccines, said Professor Klaus Cichutek, president of the Paul Ehrlich Institute. He described the high requirements for approval and the testing of each batch of the vaccine by the Paul Ehrlich Institute. Studies, he said, had been carried out with tens of thousands of participants in various countries, especially those particularly affected by corona. "This is good quality," said Cichutek. Both vaccines showed comparably high levels of efficacy at about 95 per cent and are very well tolerated. However, vaccinations should be postponed if someone is acutely ill and has a fever, for example.

What does the government say about the vaccination campaign?
The largest vaccination campaign in Germany's history has got off to a good start, Federal Health Minister Jens Spahn said in a government statement in the German Bundestag on Wednesday. "We will probably be able to offer everyone vaccination by the summer," Spahn said. However, the scarcity of vaccines worldwide at present is simply a fact, he said. The reason for this shortage is a lack of production capacities, not a lack of contracts.
The Federal Government is continuing to do everything it can to increase the availability of vaccines. Now it will also depend on whether people in Germany are willing to be vaccinated. This "largest vaccination campaign in our history" is a joint task. "Only if the vast majority of citizens are willing to be vaccinated throughout the year will we really be able to defeat the virus," Spahn emphasised.
Why is Germany coordinating its approach with its partners within the EU?
Federal Health Minister Jens Spahn stressed that it was right to go the European way in vaccine procurement. "Let me say clearly: yes, it is right that we act European." The European Union and Germany had supported the vaccine producers at a time when it was not clear for a long time who would develop an effective vaccine and which would then be approved. "Without this help, the rollout of vaccinations in Germany and Europe would hardly have been possible."
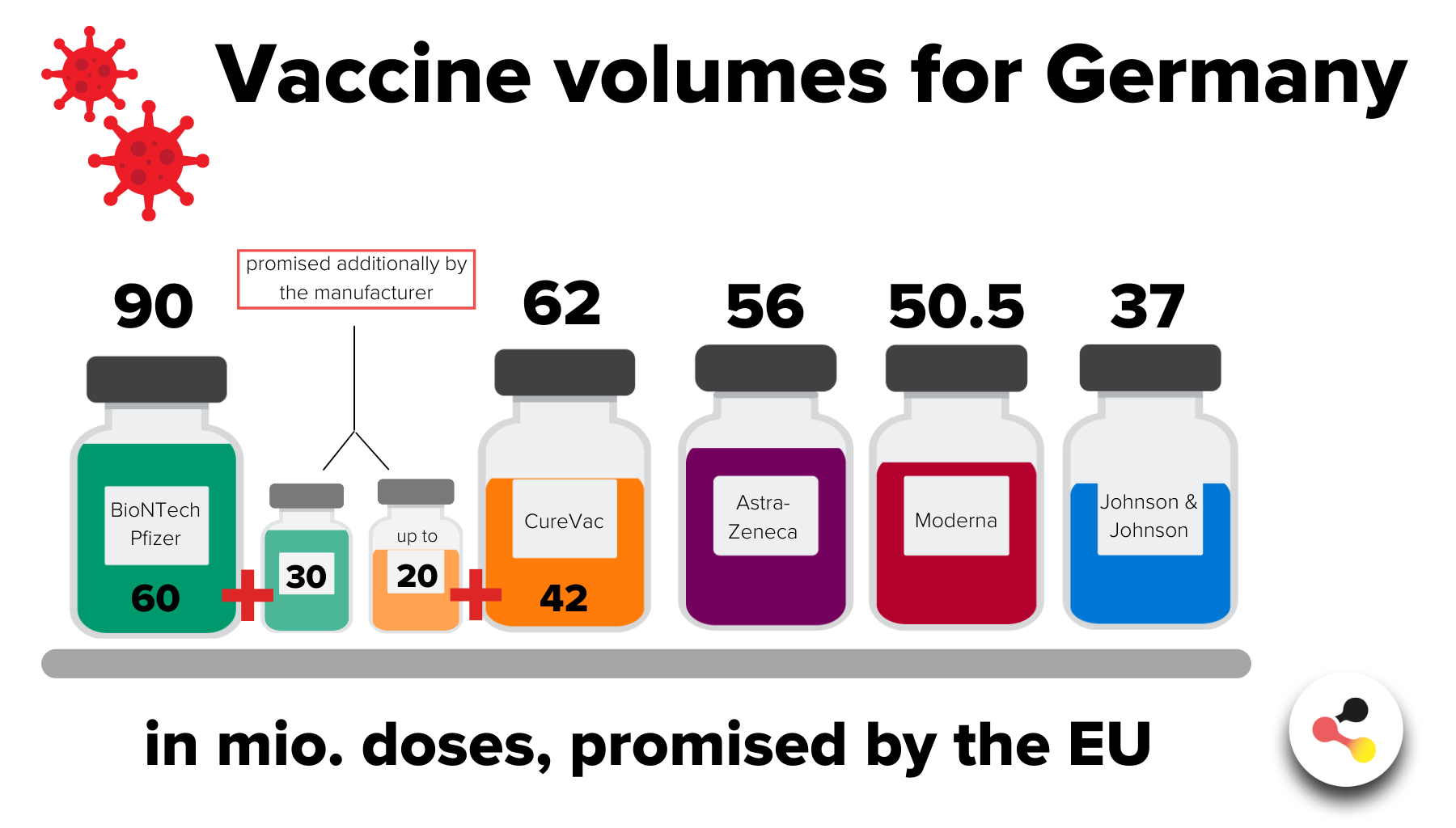
What is the current status of the various projects? What quantities of vaccine will Germany receive?
Two corona vaccines have already been approved and others may follow in the course of the year. Some projects are already at an advanced stage. Germany has secured large quantities of both approved vaccines as well as of promising candidates. An overview of the current development status:
- Biontech/Pfizer: at least 60 million doses via the EU and an assured option for a further 30 million doses nationally. (current status: vaccine approved)
- Moderna: 50.5 million doses via the EU, with additional doses being negotiated nationally. (current status: vaccine approved)
- AstraZeneca: 56 million doses via the EU(current status: vaccine approved)
- Johnson&Johnson (developer: subsidiary Janssen): 37 million doses via EU(current status: first data packages of pivotal phase three trial under review by EMA; no marketing authorisation application submitted yet)
- CureVac: at least 42 million doses via the EU and an option for 20 million doses nationally.(current status: pivotal phase-three trial started in December; no EMA review yet)
On behalf of the member states, the EU Commission has concluded central contracts with manufacturers. In times of a global pandemic, unilateral national efforts prevent effective health protection. "We have deliberately chosen a joint approach with our partners in the European Union," emphasises government spokesperson Steffen Seibert.
The European Medicines Agency (EMA) provides a comprehensive overview of the current development status of a whole range of corona vaccines.

How is Germany funding research?
Vaccinations are the key to containing the corona pandemic. That is why the Federal Government provided more than one billion euros to fund the national and international development of vaccines in 2020. "We need effective and available vaccines to overcome the pandemic, but we also need effective and available medicines to treat people who are already ill," said Federal Research Minister Anja Karliczek, talking about the research programme Funding announcement for the development of drugs and other treatments for COVID-19. The programme focuses on the development of new drugs to treat Covid-19.
In a first stage, the Federal Ministry of Education and Research had launched a basic research programme in March 2020. Its priority was to study the Covid 19 pathogen and assess the effectiveness of existing approved drugs.
